|
|
The use of single crystalline electrodes is crucial for an
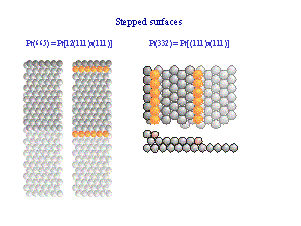
atomistic understanding. In many cases we use stepped surfaces because - they mimic active sites in heterogeneous catalysis -they allow to prepare ordered bimetallic surfaces by decoration of the steps with a second metal The use of nanoparticulate catalysts is crucial for - an understanding of particle size effects - obtaining a high surface area to (noble metal) catalyst mass ratio |
| Click here to enlarge. |
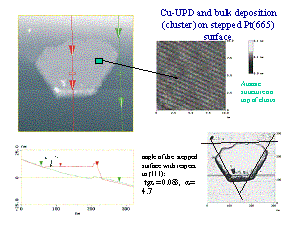
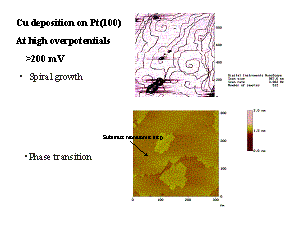
| Metal deposits on a foreign metal substrate play an important
role in industry (galvanisation). When the radii of the deposited atoms
greatly differ from those of the substrate, the deposit forms 3
dimensional clusters. When the radii coincide, deposition occurs in a
“layer by layer” mode. When the rations are close, but not identical,
the growth mode seems to depend on the surface orientation. |
|
|
Organic adsorbates are used as
modifiers in electrodeposition (galvanic
industry) and as corrosion inhibitors. They also occur as intermediates
in electrocatalysis.
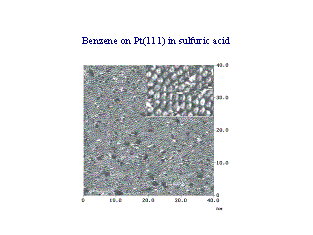 |
| In many cases the organic species can be desorbed either by
hydrogenation, displacement by other adsorbing species or by oxidation
to CO2. Using differential electrochemical mass spectrometry
(DEMS), the product can be quantitatively detected. |
Well
Structured Modification
|
Well prepared single crystals with a surface plane parallel to a highly indexed miller plane exhibit monoatomic high steps. These step sites
differ in their properties from sites within atomically smooth planes.
In general they are catalytically more active. As a consequence
|
Fuel
cell catalysis and their action
|
For the low temperature, PEM fuel cell there are two basic topics related to electrocatalysis which need improvements:
For
both anode and cathode one tries to optimize the catalysts by alloying
Pt (which in both cases is the best catalysts from all pure metals)
with other components (bimetallic and trimetallic
catalysts).
|
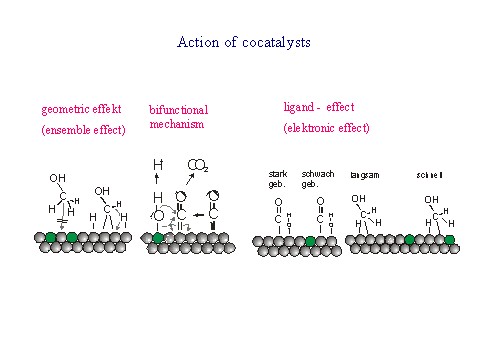  |
| The action of such co- cataysts is elucidated by model surfaces. In our
groups we use step decoration in order to obtain an ordered array of
the two components. |
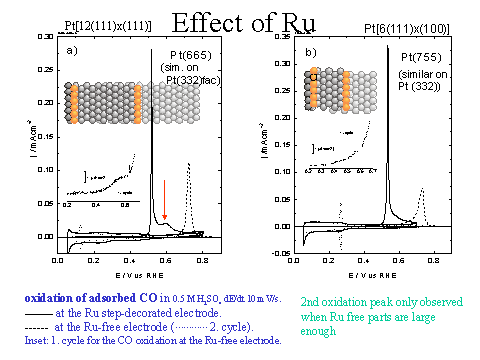
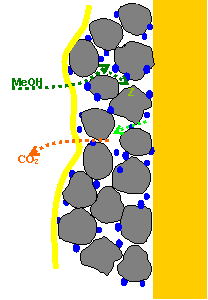
Example: CO oxidation a Ru
modified surface.
|
|
Interpretation: On Pt (665)/Ru
all adsorbed CO molecules are oxidized at a lower potential than on
pure Pt because they can diffuse to the Ru
sites where they react with oxygen species adsorbed on Ru. In addition, CO molecules adsorbed in the
vicinity of Ru are oxidized even earlier
because they are less stable adsorbed due to an electronic affect. |
|
Die Empfindlichkeit wird bestimmt durch die Diffusionsgeschwindigkeit in der dünnen Elektrolytschicht zwischen Gasphase und der Sensorelektrode. Die Ansprechzeit wird dagegen bestimmt durch Ad- bzw. Absorptionsvorgänge beim Transport in der Gasphase, insbesondere in der PTFE-Membran der Gasdiffusionselektrode. Solche Sorptionsvorgänge sind auch für alle anderen Sensortypen für organische Dämpfe im Bereich kleinster Konzentrationen bedeutsam. Die Arbeiten wurden von der Arbeitsgemeinschaft industrieller Forschungsvereinigungen „Otto von Guericke“ e.V. im Rahmen des Forschungsvorhabens 12656 N (Elektrochemische Sensoren zum Nachweis organischer Luftschadstoffe durch Adsorption) aus den Mitteln des Bundesministeriums für Wirtschaft und Arbeit (BMWA) gefördert. Die Ergebnisse wurden vorgetragen auf dem International Symposium on Instrumentalized Analytical Chemistry and Computer Technology (Incom), Düsseldorf, 29. - 31. März 2005. Ausführliche Fassung (PDF): Sensor für aromatische Kohlenwasserstoffe: Wovon hängen Empflindlichkeit und Ansprechzeit ab? |